Related Technical Articles
Introduction
What are Total Dissolved Solids (TDS)?
Types of Filtration Techniques
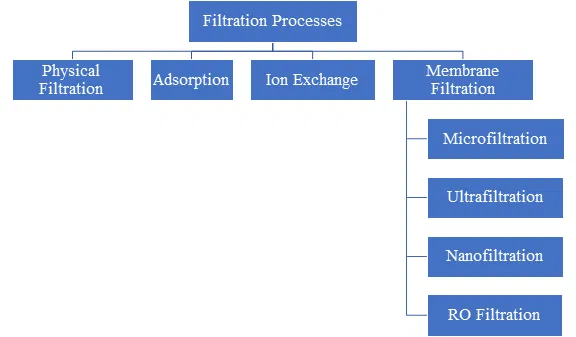
Fig 2: Types of Filtration Processes used in Drinking Water Purification
Physical Filtration
In Physical Filtration, particulates & suspended solids are removed from the inlet water through use of a physical barrier or medium. This medium can vary in shape, size, pore size & is usually dependent on the size & physical properties of the contaminants in the water. It does not involve any chemical treatment to the inlet water.
It consists of 4 stages –
- Pre-filtration (Screening),
- Sedimentation,
- Main Filtration (Sand/cartridge/Ceramic) &
- Post Filtration (Membrane)
It removes contaminants like leaves, twigs, large dense particles, bacteria, protozoa, particulates etc.
It is widely used in household & industrial water treatment systems as the first step of purification.
It enhances the chemical or biological filtration processes when water is pre-treated by this method.
It effectively removes suspended solids, sediments & particulate matter from water, does not remove essential minerals, does not rely on chemicals, does not introduce harmful chemicals, requires minimal energy input, produces minimal waste, is scalable, is durable, can last for long periods, can be effectively combined with other water treatment methods & is very simple to design, install & maintain.
However, it is ineffective in removing dissolved chemicals, salts, minerals, bacteria & virus from water, can clog with particulates & biological growth, can be breeding ground for bacteria, virus & other microorganisms, needs regular maintenance, cleaning, back-washing & media replacement, may get affected by inlet water quality thus offering a finite lifespan.
Adsorption
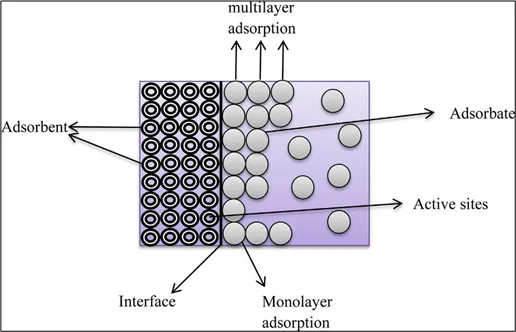
Fig 3: Adsorption process
The impurities (adsorbate) are adsorbed on the surface (adsorbent) using some materials like activated carbon, zeolites, or other porous substances.
It works on 3 principles –
- Surface Interaction: Contaminants in water come into contact with the adsorbent material.
- Binding: Contaminants bind to the surface of the adsorbent through physical forces (like van der Waals forces) or chemical interactions.
- Removal: Once bound to the adsorbent, the contaminants are effectively removed from the water, resulting in cleaner water.
It removes contaminants like chlorine, organic compounds, odors, heavy metals, ammonium ions.
It is used in household filters, industrial water treatment & municipal water treatment systems.
It enhances the effectivity & the efficiency of the purifier systems, is easy to set up & maintain with low operational cost.
However, there is a high possibility that the adsorbents (surfaces) may get saturated with the adsorbates (contaminants) needing replacement or regeneration of the systems.
Ion Exchange
In 1850, Thomas and Way performed some of the first scientific research that indicated the existence of an ion exchange process.
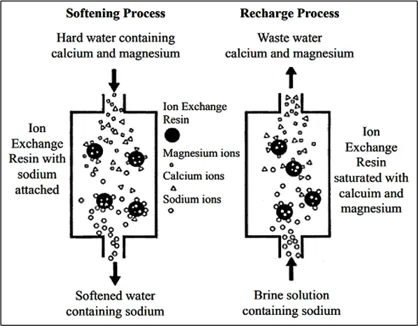
The Oxford definition of Ion Exchange is – The exchange of ions of the same charge between an insoluble solid and a solution in contact with it, used in water-softening and other purification and separation processes (Fig 4).
As a universal solvent, water contains some trace levels of gases, salts or minerals that come from the storage container or the atmosphere. When acidic rain water is neutralized by alkaline soil strata, natural salts are formed. When salts dissolve in water, they separate into charged particles called ‘ions’. A positively charged particle is a ‘cation’ (Ca2+, Mg2+, Na1+) and a negative ion is an ‘anion’ (Cl1-, NO31-, SO42-). The number of charges on an ion indicates the number of ‘counter-ions’ it will share in any reaction.
It requires use of resin beds which are functionalised porous structures or gel polymers. These beds are charged with specific anions or cations. When water is passed through these beds, the unwanted ions in water are attracted to the ions on the beds resulting in exchanges with ions on then resin. The resin saturates & needs regeneration. Regeneration of the resin beds is done by using a strong salt solution (brine) which flushes out the unwanted ions & replenishes the beds with the required ions.
It effectively removes calcium ions, magnesium ions, heavy metals & other dissolved salts.
It is used in softening, demineralisation or deionisation of water, purification of chemicals, removal of heavy metals & separation of substances.
It removes specific ions effectively resulting in high purity water & the resin bed is regenerable & reusable.
However, it may result in extra maintenance & challenge in disposal of brine solutions.
Membrane Filtration
Membrane Technology can be dated back to 1900. In 1918, Nobel Prize Winner-Professor Richard Zsigmondy & Dr Bachmann devised a method to manufacture membrane filters with consistent & reproducible porosity. In 1927, Florenz Sartorius commercially produced membranes using Zsigmondy technique.
Membrane Filtration is defined as a process that uses a selective barrier, called a membrane, to separate biomolecules and particles based on their size, allowing smaller molecules to pass through while retaining larger ones.
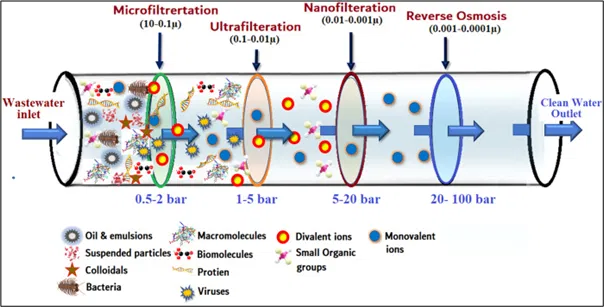
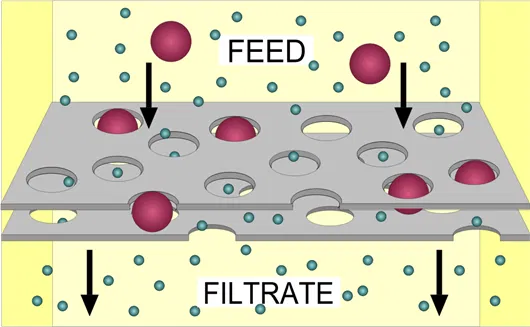
It uses pressure mechanism in which the water is forced through a semi-permeable membrane. The contaminants get trapped on the surface of the membrane & the clear water passes through. The pressure & membrane pore size depend on the types of contaminants to be removed from water (Fig 5).
It is used in water treatment, chemical and metal processing, pharmaceuticals, biotechnology, food industry, removal of environmental pollutants. It is useful to remove particles, dissolved salts, organic molecules, divalent ions, bacteria, virus, suspended solids, colloids etc.
Filter membranes are divided into four classes according to pore size:
Table 1: Different types of Membrane Filtration based on pore size & contaminants removed
Pore size | Process | Contaminants removed |
> 0.1 μm | larger bacteria, yeast, particles | |
100-2 nm | bacteria, macromolecules, proteins, larger viruses | |
2-1 nm | viruses, 2- valent ions | |
< 1 nm | salts, small organic molecules |
It is efficient in removing wide range of contaminants, has versatility of applications & scalability of use in domestic or industrial set ups.
However, it needs a specific set up which adds to the cost. It also requires regular maintenance & cleaning due to clogging & bio-fouling.
Recent Advancements In Water Treatment Technologies
Several new-generation technologies have evolved to improve efficiency, enhance contaminant removal capabilities, reduce resource cost & support sustainability namely:
- Advanced membranes made of graphene oxide or aquaporin,
- Nano-enhanced filtration with nanomaterials like carbon, silver or titanium dioxide for microbe control or electrospun nanofibers
- Hybrid Filtration Systems with Carbon-UV-UF-RO or photocatalytic filters
- Smart Filters enabled with sensors & IoT
- 3D printed or Nano-Enhanced Ceramic Filters
- Self-cleaning & Anti-fouling filters with antimicrobial coatings
- Solar-powered filters, Compact & Portable Filters, Biodegradable Filters, Recyclable Filters
Ensuring safe drinking water requires a comprehensive use of multiple technologies developed so far. At NICHEM, we mindfully work in refining the purification media used for contaminant removal as well as water enrichment.
About NICHEM
Long-standing Specialty Chemicals player with ISO 9001:2015 certification and a history of providing specialty solutions for over 25 years. The company is headed by senior chemical industry specialists with the combined expertise of more than 100 years. With an emphasis on eco-friendly, non-toxic products, the company’s primary strength is research, development, and customization. More information on NICHEM can be found at https://nichem.solutions.
References:
- https://www.britannica.com/science/filtration-chemistry
- Article on “Water treatment solution: Filtration”, retrieved on 15 October 2013 from http://www.lenntech.com/chemistry/filtration.htm
- https://en.wikipedia.org/wiki/Adsorption#cite_note-11
- Guruge, Amila Ruwan (2021-02-17). “Absorption Vs Adsorption”. Chemical and Process Engineering. Retrieved 2023-11-26.).
- https://www.researchgate.net/figure/Schematic-diagram-of-the-adsorption-process_fig3_343224678
- https://sswm.info/sswm-university-course/module-6-disaster-situations-planning-and-preparedness/further-resources-0/ion-exchange
- https://wqa.org/resources/ion-exchange/
- https://en.wikipedia.org/wiki/Ion_exchange
- https://sswm.info/sswm-university-course/module-6-disaster-situations-planning-and-preparedness/further-resources-0/ion-exchange
- https://www.researchgate.net/profile/Maik-Jornitz/publication/11205193_Sterile_filtration_-_A_review_of_the_past_and_present_technologies/links/0f31752f8d94e9b635000000/Sterile-filtration-A-review-of-the-past-and-present-technologies.pdf.
- https://watertp.tech/technologies/membrane-filtration/
- https://www.sciencedirect.com/topics/chemistry/membrane-filtration.
- https://en.wikipedia.org/wiki/Membrane_technology.
- https://www.sciencedirect.com/science/article/abs/pii/B9780323908931000076
- https://www.water-technology.net/features/latest-water-purification-technologies-top-five/