Related Technical Articles
Speciality chemicals, or performance chemicals, are meticulously formulated molecules intended to execute specialised functions in industrial and consumer products. In contrast to bulk chemicals that serve basic industrial needs, specialty chemicals are designed to deliver specific, high-performance outcomes in smaller, more targeted applications. They are utilised in diverse industries including polymers, paints & coatings, pharma, material sciences, electronics, food, water treatment, cosmetics, home care, textiles, disinfection, agriculture and more.
Simultaneously, nanotechnology, which involves working with materials at the nanoscale (1–100 nanometers), has emerged as a major achievement in materials research. Nanotechnology, when integrated with specialty chemicals, facilitates novel features and enhanced performance. Materials, when reduced to the nanoscale, exhibit drastically altered properties compared to their bulk form. For instance, titanium dioxide (TiO₂) and zinc oxide (ZnO) are usually opaque in bulk, but become transparent at the nanoscale. This illustrates how nanonisation unlocks new functionalities. This integration enables the development of sophisticated formulations that are more efficient, versatile, and better aligned with contemporary industrial requirements. Collectively, these disciplines are influencing the forthcoming generation of advanced materials.
What Are Nanomaterials?
Nanomaterials are materials with structural components that measure between 1 to 100 nanometers (nm) in at least one dimension. To understand the scale, 1 nanometer is one-billionth of a meter, about 100,000 times smaller than the width of a human hair.
At this extremely small size, materials exhibit unique physical, chemical, electrical, and optical properties that differ significantly from their bulk counterparts. These changes are primarily due to:
- High surface area-to-volume ratio
- Quantum effects that affect electron behaviour
Surface reactivity and energy that influence interactions with surrounding molecules
Nanomaterials may exist in different forms as depicted in the following Fig. 1:
- Nanoparticles (0D): They are roughly spherical or irregular
- Nanorods/Nanowires (1D): They are elongated structures with nanoscale
- Thin films (2D): These are flat layers used in coatings and
- Bulk nanomaterials (3D): These are materials with nanoscale features
Fig 1. Types and Shapes of Nanoparticles (Viegas, C., et al., 2022)
Nanomaterials are characterized using advanced instruments that can detect and measure features at the nanoscale. Examples of such advanced instruments are listed in Table 1.
Table 1. Analytical Instruments to Measure Nanoparticle Size
Instrument | Principle | Use |
Transmission Electron Microscopy (TEM) | Uses electron beams to visualise particles at atomic resolution | Shape, size, internal structure |
Scanning Electron Microscopy (SEM) | Electron beam scans the surface to form 3D-like images | Surface morphology |
Dynamic Light Scattering (DLS) | Analyses light scattering due to Brownian motion | Size distribution in suspensions |
Atomic Force Microscopy (AFM) | Measures surface topography using a fine probe | 3D mapping, especially of soft materials |
X-Ray Diffraction (XRD) | Measures crystalline structure, calculates particle size via the Scherrer equation. | Crystal size and phase |
Which Chemicals/Elements Are Used As Nanomaterials?
Nanomaterials are engineered from a wide variety of chemical elements and compounds. They are chosen based on the desired properties, optical, electrical, catalytic, mechanical, or biological. These materials are either inorganic, organic, or hybrid in nature and can be customised through precise control of size, shape, and surface chemistry. The most commonly used nanomaterials are covered in Table 2 below:
Table 2. Chemicals and Elements Commonly Used as Nanomaterials
Category | Nanomateri al | Key Properties | Common Applications | Nanoparticle size range (nm) |
Metal Nanoparticles | Silver (Ag) | Antimicrobial, conductive, surface-reactive | Disinfectants, textiles, home care, wound dressings | 1–100 |
Gold (Au) | Biocompatible, optical tunability, stable | Diagnostics, drug delivery, biosensors | 1–100 | |
Platinum (Pt) | Excellent catalytic efficiency, redox stability | Emission control, fuel cells, chemical manufacturing | 1–10 | |
Palladium (Pd) | Hydrogen absorption, catalytic activities | Automotive catalysts, hydrogen sensors, specialty catalysis | 2-50 |
| Copper (Cu) | Antimicrobial, good conductor, cost-effective | Electrical components, coatings, paints | 2-100 |
Metal Oxide Nanoparticles | Titanium Dioxide (TiO₂) | UV absorption, photocatalytic, self-cleaning | Sunscreens, paints, antimicrobial coatings | 5-100 |
Zinc Oxide (ZnO) | UV blocking, antimicrobial, semiconducting nature | Cosmetics, sunscreens, surface coatings | 10-100 | |
Iron Oxide (Fe₃O₄/Fe₂O₃ ) | Magnetic, biocompatible | MRI imaging, water treatment, data storage | 5-50 | |
Cerium Oxide (CeO₂) | Oxygen buffering, redox cycling, radical scavenging | Emission control, polishing agents, catalytic converters | 3-50 | |
Aluminum Oxide (Al₂O₃) | Hard, thermally stable, insulating | Abrasives, coatings, ceramics | 20-100 | |
Silicon Dioxide (SiO₂) | Chemically inert, high surface area, porous | Paints, cosmetics, food additives, pharmaceutical excipients | 5-100 | |
Carbon-Based Nanomaterials Natural/Comp osite Nanomaterials | Carbon Nanotubes (CNTs) | High strength, electrical/thermal conductivity | Polymer composites, electronics, aerospace components | Diameter: 1–50, Length: up to microns |
Graphene | Exceptional conductivity, thermal management | Batteries, sensors, conductive films | 1–10 (thickness), lateral size 100–1000+ |
| Fullerenes (C₆₀) | Antioxidant, cage structure, electron acceptor | Skincare, anti-aging products, and drug delivery | ~0.7 |
Zeolites | Crystalline, porous, ion exchange capacity | Catalysis, water purification, and slow-release fertilisers | 10–200 | |
Micro Silver | Controlled ion release, long-term antimicrobial effect | Home care, wound healing, disinfectant formulations | 50–1000 | |
Chitosan/Alg inate | Biodegradable, mucoadhesive, non-toxic | Drug carriers, seed coating, and agricultural sprays | 10–500 |
Adapted from: Altammar, K. A. Front. Microbiol., vol. 14, 2023.
Applications of Nanoparticles in Speciality Chemicals
The performance of nanoparticles is closely tied to three structural factors:
- Size: Smaller particles offer greater surface area per unit volume, improving interaction with substrates or reactants.
- Shape: Rods, tubes, spheres, and sheets behave differently in fluid matrices, influencing dispersibility, reactivity, and mechanical reinforcement.
- Surface Chemistry: Functional groups on nanoparticle surfaces can be tailored to enhance compatibility, adhesion, or targeting in chemical formulations.
These structural attributes, such as size, shape, and surface chemistry, are not merely scientific considerations. They serve as design levers for creating high-performance specialty chemical solutions. Industries now formulate with intent, choosing nanoparticle types that align with the end-use requirement. For example, coatings incorporate nanoparticles not just for UV protection but to engineer surfaces that repel dirt or inhibit microbial growth. This targeted use of nanotechnology is shifting formulations from being functional to smart.
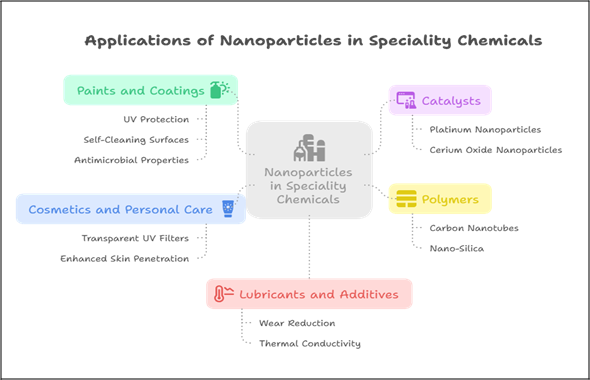
Fig 2. Applications of Nanoparticles in Speciality Chemicals
The sector-wise applications of nanoparticles are discussed as follows:
1. Paints and Coatings
Nanoparticles are transforming the performance of paints and coatings, particularly for surfaces exposed to sunlight, pollution, and microbes. Two materials, titanium dioxide (TiO₂) and zinc oxide (ZnO), are frequently used for these purposes.
UV Protection
TiO₂ and ZnO nanoparticles serve as highly efficient UV blockers. Their nanoscale size allows them to scatter and absorb harmful ultraviolet radiation without affecting the clarity or colour of the coating.
This protection helps preserve the underlying materials, such as exterior walls, wood panels, metals, or synthetic surfaces. It works by preventing fading, chalking, and degradation caused by prolonged sun exposure. These nanoparticles are especially useful in architectural coatings, automotive finishes, and marine paints.
Self-Cleaning Surfaces
Titanium dioxide also acts as a photocatalyst. Under UV light, it generates reactive oxygen species (ROS) that break down organic dirt, grime, and airborne pollutants. Its hydrophilic surface allows rainwater to spread evenly and carry away the degraded contaminants, reducing the need for manual cleaning. This feature is used in building façades, glass coatings, and solar panels to maintain cleanliness with minimal maintenance.
Antimicrobial Properties
ZnO nanoparticles are known for their antimicrobial activity. They release zinc ions and generate ROS, both of which disrupt bacterial membranes and metabolic pathways. Coatings enhanced with ZnO are now used in hospital walls, food packaging, and public transport surfaces where hygiene is a concern.
2. Catalysts
Nanoparticles are widely used as catalysts because of their high surface area and ability to participate in redox reactions more efficiently than bulk materials.
Platinum Nanoparticles
In catalytic converters, platinum nanoparticles accelerate the oxidation of carbon monoxide (CO) and unburned hydrocarbons (HC) into less harmful carbon dioxide (CO₂) and water vapour. Their finely dispersed structure means more active sites are available for reaction, which promotes faster and more complete conversion.
Reaction example:
- CO + ½O₂ → CO₂
- CₓHᵧ + O₂ → CO₂ + H₂O
Cerium Oxide Nanoparticles (CeO₂)
Cerium oxide is another crucial catalyst in automotive applications. Its ability to switch between Ce³⁺ and Ce⁴⁺ oxidation states enables it to store and release oxygen, which helps maintain optimal oxygen levels in the exhaust system. This property enhances the conversion of nitrogen oxides (NOₓ) into nitrogen (N₂) and oxygen.
3. Polymers
Adding nanoparticles to polymer systems improves their durability, strength, and heat resistance without increasing weight.
Carbon Nanotubes (CNTs)
CNTs are incorporated into thermoplastics and thermosets to increase tensile strength and improve thermal and electrical conductivity. These composites are now common in aerospace panels, high-performance sporting goods, and electronic casings. The tubular structure of CNTs helps reinforce the polymer matrix by resisting crack propagation.
Nano-Silica
Silica nanoparticles improve polymer toughness and thermal stability. They also enhance gas barrier properties, which is valuable in food packaging films, where oxygen and moisture resistance is needed. Their spherical shape contributes to better dispersion and integration into polymer chains.
4. Cosmetics and Personal Care
Nanoparticles in personal care products are used to improve UV protection, sensory feel, and the delivery of active ingredients.
Transparent UV Filters
Nano-TiO₂ and Nano-ZnO are commonly used in sunscreens. Unlike their larger counterparts, these nanosized particles provide strong UVA and UVB protection without leaving a white residue. Their ability to remain transparent on the skin while blocking harmful radiation makes them preferred ingredients in modern formulations.
Enhanced Skin Penetration
The nanoscale size of delivery vehicles, such as liposomes or nano-emulsions, allows active compounds, like vitamins, peptides, or antioxidants, to penetrate deeper into the skin layers. This enhances the effectiveness of moisturisers, anti-ageing serums, and treatment products.
5. Lubricants and Additives
Nanoparticles are being explored in advanced lubricants to reduce mechanical wear and improve heat management in engines and machinery.
Wear Reduction
Metal oxide nanoparticles, such as ZnO or Al₂O₃, form a thin, protective layer on metal surfaces under friction. This layer reduces direct contact between surfaces, minimising wear and extending the lifespan of engine components. These nanoparticles act like tiny ball bearings, improving lubrication under high stress.
Thermal Conductivity
Some nanoparticles also improve the heat dissipation of lubricants. For example, copper oxide or carbon-based nanoparticles increase the thermal conductivity of the oil, allowing heat to move away from critical areas more efficiently. This prevents overheating and improves engine performance under load.
Conclusion
Nanotechnology is redefining how specialty chemicals are formulated and applied across industries. By operating at the nanoscale, materials like TiO₂, ZnO, CNTs, and metal oxides exhibit enhanced properties, greater reactivity, UV protection, antimicrobial action, and improved mechanical strength. These features are used in paints, coatings, cosmetics, catalysts, polymers, and lubricants to create smarter, more durable, and efficient products. Each application shows how the size, shape, and surface chemistry of nanoparticles are intentionally selected to deliver targeted performance. This convergence of nanoscience and specialty chemistry marks a shift from conventional formulation to precision engineering at the molecular level.
Want to explore the advantages, action mechanisms, challenges, and future prospects of using nanoparticles in specialty chemicals? Check out the next article to get the full picture.
References:
- Tadesse S, Hailemariam T. A review on the classification, characterisation, synthesis of nanoparticles and their application. Advances [Internet]. 2025 Jun 23;6(2):63–72. Available from: https://doi.org/10.11648/j.advances.20250602.15
- Nanotechnology in the world of paints and coatings [Internet]. American Coatings Association. Available from:http://www.paint.org/coatingstech-magazine/articles/nanotechnology-in-the-world-of-paints-a nd-coatings/
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari M, et al. Nanotechnology in Cosmetics and Cosmeceuticals—A review of latest advancements. Gels [Internet]. 2022 Mar 10;8(3):173. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8951203/
- Study Mind. Advantages & Risks of nanoparticles (GCSE Chemistry) [Internet]. Study 2024. Available from: https://studymind.co.uk/notes/advantages-risks-of-nanoparticles/
- Applications of Nanomaterials in Chemical Industry.” Futuristic Trends in Chemical, Material Sciences & Nano Technology, vol. 3, Iterative International Publisher (IIP), May 2024, 68–79. Available from:https://www.researchgate.net/publication/380324662_APPLICATIONS_OF_NANOMATERIALS_IN_CHEMICAL_INDUSTRY
- Specialty Chemicals: an emerging technology [Internet]. Signicent LLP. 2025. Available from https://signicent.com/specialty-chemicals-an-emerging-technology
- Szczyglewska P, Feliczak-Guzik A, Nowak I. Nanotechnology–General aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules [Internet]. 2023 Jun 22;28(13):4932. Available from: https://www.mdpi.com/1420-3049/28/13/4932