Related Technical Articles
Nanoparticles in Specialty Chemicals: Advantages, Mechanisms, and Future Prospects
Nanomaterials exhibit physicochemical properties different from their bulk counterparts. However, their performance within real-world formulations is not solely governed by their intrinsic properties. Instead, it is shaped by factors such as dispersion behaviour, surface chemistry, compatibility with surrounding matrices, and processing conditions.
Formulators may notice that the same nanoparticle, such as titanium dioxide, fumed silica, or a layered silicate, can function effectively in one system but underperforms or destabilises another. These inconsistencies are not anomalies but are indicative of how formulation and nanoparticle interactions govern the final outcome. Parameters additive selection, solvent polarity, and even the sequence of ingredient addition can significantly influence performance.
If you’re looking to design more responsive, durable, or targeted formulations, keep reading to learn their working mechanisms, key production methods, practical advantages, and what’s limiting wider adoption.
Advantages of Using Nanoparticles
Nanoparticles offer unique advantages in specialty chemical formulations across industries, as depicted in Fig 3:
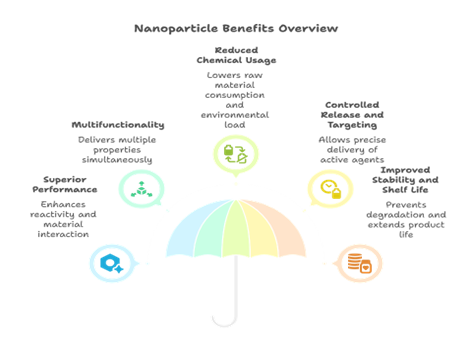
Fig 3. Advantages of Using Nanoparticles
Superior Performance
Their high surface-area-to-volume ratio improves reactivity and material interaction. This enhances product performance at lower loading concentrations. In coatings, for example, nanoparticles boost UV resistance and film durability. In catalysts, they increase reaction rates and lower activation energies.
Multifunctionality
A single nanoparticle can deliver multiple properties simultaneously. For instance, ZnO offers UV shielding and antimicrobial effects together. Carbon nanotubes improve both tensile strength and electrical conductivity. Such combinations reduce the need for multiple additives in formulations.
Reduced Chemical Usage
Due to their efficiency, lower quantities of active ingredients are needed. This results in reduced raw material consumption and lower environmental load. It also helps in minimising toxicity and waste in downstream processes.
Controlled Release and Targeting
Surface-modified nanoparticles allow controlled release of active agents. This is particularly useful in agriculture and personal care formulations. Targeting ability also enhances therapeutic precision in biomedical applications.
Improved Stability and Shelf Life
Nanoparticles can improve formulation stability in harsh conditions. They help prevent oxidation, microbial growth, and degradation in storage. This is valuable in paints, cosmetics, and home care products.
Production Strategies of Nanomaterials
Nanomaterials are manufactured using two main approaches, as depicted in Fig 4:
- Top-down
- Bottom-up
Top Down Approach
In top-down approaches, bulk materials are broken down into nanoscale structures using mechanical or physical techniques. Common methods include mechanical milling, laser ablation, and lithography.
While these techniques are effective for producing nanoparticles in large volumes, they may introduce surface defects or irregular shapes.
Bottom Up Method
Bottom-up approaches build nanoparticles atom-by-atom or molecule-by-molecule. These include chemical vapour deposition (CVD), sol-gel synthesis, co-precipitation, and hydrothermal methods. These techniques allow precise control over particle size, shape, and composition, often resulting in more uniform and defect-free nanomaterials. Biological or green synthesis is also gaining popularity, using plant extracts or microbes as eco-friendly reducing agents.
The choice of method depends on the desired properties, material type, and application. For example, metal oxides for coatings, carbon nanotubes for polymers, and quantum dots for imaging each require specific synthesis techniques. Scalability, purity, and cost-efficiency also influence the manufacturing route.
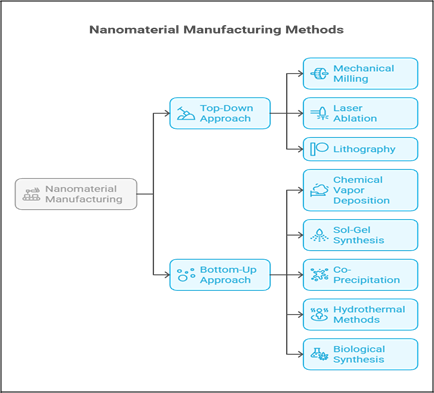
Fig 4. Nanomaterial Manufacturing Methods
Mechanism of Nanoparticle Action
At the nanoscale, the dominant forces governing material behaviour include van der Waals interactions, electrostatic forces, and surface energy effects. Due to their small size and large surface area, nanoparticles exhibit:
- Surface Reactivity: Atoms on the surface are unsaturated, which increases interaction with surrounding molecules.
- Quantum Effects: Electrons in nanoparticles are confined, which affects their optical and electronic behaviour.
- Localised Effects: Due to their size, nanoparticles can penetrate and interact with structures at the cellular or molecular level in biological or polymeric systems.
For example, in catalysis, nanoparticles act by providing more reactive surface sites. It reduces activation energy and enables multi-step reactions at ambient conditions. In UV filters, their ability to absorb and scatter UV radiation depends on the particle size and crystalline structure.
The mechanism of action varies based on the type of nanoparticle (metal, oxide, carbon-based, polymeric), the target system (microbial, catalytic, biological, or environmental), and the surrounding conditions (pH, temperature, redox environment). However, some generalised pathways can be described as depicted in Fig 5:
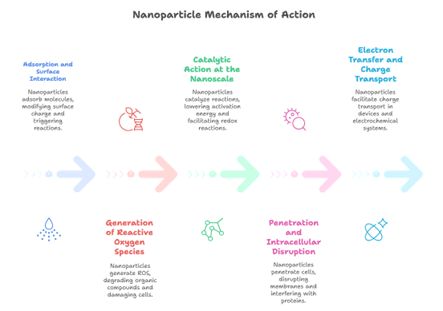
Fig 5. General Mechanism of Action of Nanoparticles
1. Adsorption and Surface Interaction
Nanoparticles possess highly active surfaces that readily adsorb ions, molecules, or biomolecules. This adsorption can:
- Modify surface
- Influence dispersion and aggregation
- Trigger catalytic reactions at the
For instance, TiO₂ and ZnO nanoparticles adsorb organic molecules and pollutants on their surfaces before degrading them through photocatalysis.
2. Generation of Reactive Oxygen Species (ROS)
A critical mechanism, especially for metal oxide nanoparticles, involves the generation of reactive oxygen species (ROS) such as hydroxyl radicals (•OH), superoxide anions (O₂⁻•), and hydrogen peroxide (H₂O₂). These species are highly reactive and can degrade organic compounds, damage microbial membranes, or initiate oxidation reactions.
Example with TiO₂ under UV light:
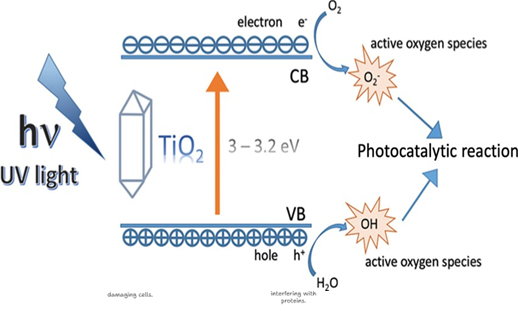
Fig 6. Mechanisms of UV and visible light activation of TiO2
When titanium dioxide (TiO₂) nanoparticles are exposed to ultraviolet (UV) light, they undergo a photocatalytic activation process, as shown in Fig 6. This process begins when TiO₂ absorbs photons with energy equal to or greater than its band gap (~3.2 eV for the anatase form):
TiO₂ + hν → TiO₂ (e⁻ + h⁺)
Formation of electron-hole (e⁻/h⁺) pairs
In this step, an electron (e⁻) from the valence band (VB) of TiO₂ is excited to the conduction band (CB), leaving behind a hole (h⁺). These charge carriers migrate to the surface and drive redox reactions with surrounding molecules.
The hole (h⁺) acts as a strong oxidising agent and reacts with adsorbed water (H₂O) or hydroxide ions (OH⁻) on the nanoparticle surface to generate hydroxyl radicals (•OH):
h⁺ + H₂O → •OH + H⁺ or
h⁺ + OH⁻ → •OH
Simultaneously, the electron (e⁻) in the conduction band reduces molecular oxygen (O₂) to form superoxide anion radicals (O₂⁻•):
e⁻ + O₂ → O₂⁻•
These ROS attack cellular components, disrupt DNA, proteins, and lipids, or oxidise pollutants into non-toxic products.
3. Catalytic Action at the Nanoscale
Nanoparticles, especially noble metals like Platinum (Pt), Palladium (Pd), and Gold (Au), exhibit high catalytic activity due to their ability to:
- Lower activation
- Provide active sites for redox
- Maintain high turnover
Example: CO Oxidation using Pt nanoparticles
At the nanoscale, Pt particles present a much higher surface area-to-volume ratio than their bulk counterparts, exposing more active sites for chemical interactions. As shown in Fig. 5, this enables efficient adsorption of both CO molecules and oxygen (O₂) onto the Pt surface.
The reaction occurs via the Langmuir-Hinshelwood mechanism, where both reactants are adsorbed on adjacent sites:
- CO(g) → CO(ads)
- O₂(g) → 2O(ads)
- CO(ads) + O(ads) → CO₂(g)
Once adsorbed, oxygen molecules dissociate into reactive oxygen atoms. These atoms react with the neighbouring CO molecules to form carbon dioxide (CO₂), which then desorbs from the surface.
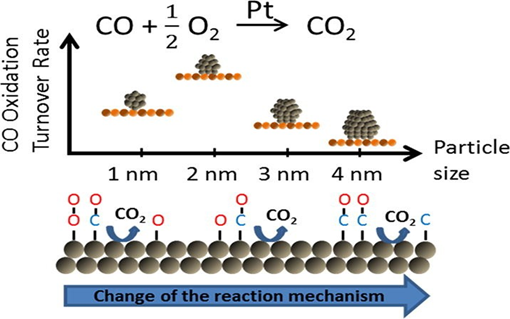
Fig 7. Catalytic Action of Pt at the Nanoscale
This process is highly efficient at lower temperatures when using Pt in nanoparticle form. Compared to bulk Pt, the nanostructured version lowers the activation energy for the reaction due to quantum-size effects and enhanced electronic interactions.
4. Penetration and Intracellular Disruption (Biomedical Context)
Due to their size, nanoparticles can penetrate cells and organelles, especially in bacteria or cancer cells. They can:
- Interact with DNA or
- Disrupt cell membranes through oxidative
- Interfere with protein
Silver nanoparticles, for instance, release Ag⁺ ions that bind to thiol (-SH) groups in proteins. This silver ion inhibits enzymes and leads to cell death.
5. Electron Transfer and Charge Transport
Semiconducting nanoparticles such as zinc oxide (ZnO) or graphene-based materials have unique electronic band structures with distinct VB and CB. This structure allows them to participate actively in photo-induced or electrochemical redox reactions by transferring electrons.
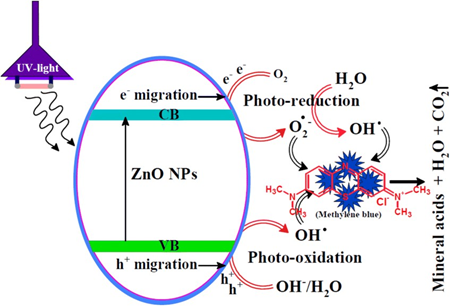
Fig 8. Schematic band diagrams of ZnO nanoparticles with charge transportation processes
As seen in Fig 8, when ZnO is irradiated with light energy ≥ its band gap (≈3.3 eV), electrons in the valence band absorb the energy and jump to the conduction band, leaving behind holes (h⁺).
ZnO + hv → ZnO (e⁻_CB + h⁺_VB)
The photo-generated electrons (e⁻) move through the conduction band. Simultaneously, holes (h⁺) migrate in the opposite direction within the valence band. Efficient charge separation and mobility reduce recombination and enable further redox reactions.
The excited e⁻ in CB reduces surface-adsorbed electron acceptors like O₂: e⁻ + O₂ → O₂⁻•
The h⁺ in VB oxidises water or hydroxide ions to generate hydroxyl radicals: h⁺ + H₂O → •OH + H⁺
In materials like graphene, which is a zero-bandgap semiconductor, high electron mobility facilitates rapid and lossless electron transport, especially in electrochemical sensors and supercapacitors. Graphene acts as a conductive channel, moving charges between redox centres and electrodes.
Challenges of Using Nanoparticles
Despite their benefits, nanoparticles present several implementation challenges.
Toxicity and Safety Concerns
Inhalation of nanoparticles may cause lung irritation or inflammation. Dermal exposure can lead to accumulation in skin or systemic circulation. Their small size allows them to cross biological barriers easily. Long-term environmental effects are not yet fully understood or documented.
Stability and Dispersion
Nanoparticles tend to agglomerate due to surface energy. Agglomeration reduces surface area and affects performance reliability. Uniform dispersion in liquids or solids requires precise formulation strategies. Use of surfactants or polymeric stabilisers is often necessary.
Cost and Scalability
Producing nanoparticles at an industrial scale remains technically demanding. Processes like sol-gel, precipitation, or vapour deposition are often costly. Maintaining particle uniformity and purity at scale is difficult. Supply chain and regulatory complexities add to commercialisation challenges.
Future of Nanoparticles in Specialty Chemicals
Innovation is driving the next generation of nanoparticle-based applications.
Smart Coatings and Self-Healing Materials
Self-healing coatings use nanocapsules that release sealants upon damage. Such systems extend product life and reduce maintenance needs. In corrosion protection, smart coatings can detect and repair microcracks. These materials adapt to environmental changes for enhanced durability.
Integration with AI and IoT
The integration of nanoparticles with AI and IoT will lead to responsive, smart materials that are capable of sensing and reacting to environmental changes like pH, pressure, or temperature. These systems can enable real-time data transmission via IoT networks, enabling applications in smart packaging, structural
health monitoring, and wearables. The intersection enables precise control and predictive maintenance across industries.
Biodegradable and Green Nanomaterials
Sustainability has become a central design principle in nanomaterial development for specialty chemicals. Traditional metal oxide nanoparticles, like TiO₂ and ZnO, though effective, often raise long-term environmental and toxicological concerns. As a response, biodegradable and bio-based nanomaterials are emerging as safer, greener alternatives.
Sustainability in Nanotechnology
Biologically sourced nanocarriers, such as chitosan, cellulose nanofibers, starch-based nanoparticles, and lipid-based vesicles, offer key sustainability advantages. They are naturally biodegradable and break down into non-toxic compounds, posing minimal risk to soil and aquatic ecosystems. These bio-based materials ensure lower ecotoxicity. Their synthesis often relies on green, sustainable methods using plant extracts, enzymes, or microbes, which operate at ambient conditions, avoid toxic solvents, and generate less waste.
Precision Agriculture and Smart Delivery
Nano-fertilisers deliver nutrients on a timed or trigger basis. Nanocarrier-based pesticides minimise chemical runoff and crop resistance. These technologies can enhance yields at the expense of less environmental degradation.
Conclusion
Nanoparticles have become critical enablers of advanced functionality in specialty chemicals. Their characteristics to manipulate interactions at the molecular level allow for improvements in efficiency, durability, and multi-functionality. From antimicrobial coatings to high-performance polymers and second-generation sunscreens, nanoparticles are facilitating a revolution in everyday product expectations.
We at NICHEM are unlocking this potential by strategically applying nanomaterials such as micro silver, zeolites, palladium, and titanium dioxide in different verticals. As the technology, formulation and safety standards are developed, nanotechnology is likely to open up new possibilities in specialty chemicals and aid in the creation of even smarter, safer, and greener products.
References:
- Tadesse S, Hailemariam T. A review on the classification, characterisation, synthesis of nanoparticles and their application. Advances [Internet]. 2025 Jun 23;6(2):63–72. Available from: https://doi.org/10.11648/j.advances.20250602.15
- Nanotechnology in the world of paints and coatings [Internet]. American Coatings Association. Available from: http://www.paint.org/coatingstech-magazine/articles/nanotechnology-in-the-world-of-paints-a nd-coatings/
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari M, et al. Nanotechnology in Cosmetics and Cosmeceuticals—A review of latest advancements. Gels [Internet]. 2022 Mar 10;8(3):173. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8951203/
- Study Mind. Advantages & Risks of nanoparticles (GCSE Chemistry) [Internet]. Study 2024. Available from: https://studymind.co.uk/notes/advantages-risks-of-nanoparticles/
- Applications of Nanomaterials in Chemical Industry.” Futuristic Trends in Chemical, Material Sciences & Nano Technology, vol. 3, Iterative International Publisher (IIP), May 2024, 68–79. Available from:https://www.researchgate.net/publication/380324662_APPLICATIONS_OF_NANOMATERIALS_IN_CHEMICAL_INDUSTRY
- Specialty Chemicals: an emerging technology [Internet]. Signicent LLP. 2025. Available from: https://signicent.com/specialty-chemicals-an-emerging-technology
- Szczyglewska P, Feliczak-Guzik A, Nowak I. Nanotechnology–General aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules [Internet]. 2023 Jun 22;28(13):4932. Available from: https://www.mdpi.com/1420-3049/28/13/4932