Related Technical Articles
How Does Silver Hydrogen Peroxide Work As A Disinfectant
Hospitals, farms, and food plants face relentless threats from bacteria, fungi, and viruses. By 2050, antimicrobial resistance could claim 10 million lives each year, making it deadlier than cancer. Moreover, many facilities still rely on chlorine or phenolics, which clear germs but leave toxic residue behind.
This is where silver-hydrogen peroxide (SHP), as a disinfectant, acts as a one-stop solution, fighting the superbug with its dual action. It is a dual-action disinfectant known for its broad-spectrum efficacy, environmentally safe profile, and ability to degrade into harmless byproducts. So, read on to learn how SHP works, its application, and use cases.
Composition and Chemistry of Silver-Hydrogen Peroxide
Before you learn about the efficacy and application of this disinfectant, let us look closely at the composition and chemical properties of SHPs:
- Chemical Structure and Formulation: SHP forms when hydrogen peroxide (H₂O₂) combines with silver ions (Ag⁺). Its molecular formula reads AgH₂O₂⁺ , and it weighs around 142 g/mol.
- Silver‑Peroxide Complex: H₂O₂ coordinates to Ag⁺ through its peroxide oxygen atoms, forming an Ag-O-O-H intermediate. This bond raises the O-O bond energy and slows spontaneous H₂O₂ breakdown.
- Intermediary Radicals (IP): In the presence of Ag⁺, the peroxide O-O Bond cleaves selectively under microbial contact to produce hydroxyl (OH) and hydroperoxyl (OOH) radicals (high‑energy species that attack cell components).
- Peroxide Activation: Silver ions act as a redox partner. They accept an electron from H₂O₂, forming Ag-HO and Ag-OOH species that convert back to Ag⁺ while releasing OH radicals.
- Silver Ion Reservoir: After radicals form, Ag⁺ remains available on It binds thiol (-SH) groups in proteins and nucleic acids, preventing microbial repair of oxidative damage. Lab tests show 4-8 mg/L kills 90% of E. coli, P. aeruginosa and S. aureus.
- Catalytic Enhancement by Silver Ions: Silver raises reactive oxygen production two to three times more in Gram-negative cells than in Gram-positive ones.
- Depot‑Storage Effect: Silver ions inhibit H₂O₂’s slow, spontaneous decomposition. In the dark, at room temperature, the complex retains over 90 % activity for 6
- Light and Heat Sensitivity: Ultraviolet (UV) or >40 °C conditions accelerate Ag-peroxide breakdown. Hence, it must be stored in opaque and cool containers.
Mechanism of Action
The unique composition of silver hydrogen peroxide, combining the catalytic power of silver and the oxidative nature of H₂O₂, provides a multi-pronged attack to eliminate microbial pathogens. Here’s a breakdown of this biodegradable disinfectant’s mechanism:
1. Coordination of H₂O₂ to Ag⁺
Ag+ + H2O2 ⇌ [Ag-OOH]+
Silver binds the peroxide via one of its O-O oxygens, raising the bond energy and “storing” the oxidant in a depot form
2. Fenton‑Like Radical Generation
[Ag-OOH]+ ⟶ Ag+ + ⋅OH + ⋅O−
This step resembles a Fenton reaction, where Ag⁺ acts as a redox catalyst to cleave H₂O₂ into hydroxyl (·OH) and superoxide‑type (·O⁻) radicals.
3. Lipid Peroxidation
⋅OH + R−CH=CH−R′ ⟶ R−C(OH)−C⋅−R′ ⟶ Membrane rupture
Hydroxyl radicals strip electrons from unsaturated membrane lipids, initiating chain reactions that compromise membrane integrity.
4. Protein and Enzyme Oxidation
⋅OH + R−SH ⟶ R−S⋅ + OH− ⟶ Enzyme inactivation
Radicals oxidize cysteine thiol groups, denaturing key metabolic enzymes and halting cellular respiration.
5. Nucleic Acid Damage
⋅OH + dG ⟶ 8‑oxo‑dG lesions ⟶ Strand breaks
Radical attack on DNA bases causes mutations and strand scission, preventing replication and transcription.
6. Silver Ion Binding to Thiols and DNA
Ag+ + R−SH ⟶ R−S–Ag + H+
Ag⁺ forms stable bonds with thiol groups in proteins, further denaturing enzymes. It also gets into nucleotide bases, blocking polymerases.
7. Oxidative Dissolution of Silver Nanoparticles
AgNP0 + H O ⟶ Ag+ + ⋅OH + OH−
Any residual Ag⁰ in colloidal form undergoes oxidative dissolution, releasing more Ag⁺ and sustaining radical generation
8. EPS Matrix Breakdown
⋅OH + (C6H10O5)n ⟶ Oligosaccharides ⟶ Matrix collapse
Radicals oxidize the polysaccharide structure of biofilms (EPS), opening channels for deeper penetration
9. Deep Layer Kill
The combination of radicals and free Ag⁺ diffuses into the loosened biofilm, killing embedded cells and preventing regrowth.
Silver-H₂O₂ outperforms traditional agents like chlorine, quats, and phenolics in biofilm disruption and residual activity, as compared below:
Disinfectant | Mechanism | Advantages | Limitations |
Silver-H₂O₂ | Oxidative damage and Ag⁺ binding | ● Synergy ● Lasting effect ● Biofilm penetration | ● Needs stabilizers ● Light/heat sensitive |
Chlorine | Oxidation via hypochlorous acid | ● Broad spectrum ● Low cost | ● Corrosive ● Harmful |
Quaternary Ammonium Compounds (Quats) | Membrane disruption | ● Surface-safe ● Low toxicity | ● Weak on spores ● Limited biofilm action |
Phenolics | Protein denaturation and membrane damage | ● Works with organic load | ● Toxic residue ● Handling risks |
Spectrum of Efficacy
The SHP shows a broad spectrum of efficacy. It ensures protection from bacteria, fungi, spores, viruses, and even multidrug-resistant strains in the following ways:
● Bactericidal (Gram-positive and Gram-negative)
Silver-H₂O₂ kills both Gram-positive bacteria like Staphylococcus aureus and Enterococcus faecalis and Gram-negative strains such as Escherichia coli and Pseudomonas aeruginosa. A study shows that it cuts bacterial counts by 99.9% (≥4-log reduction) within just 3 minutes.
● Fungicidal and virucidal properties
This mix destroys yeast cells (for example, Candida albicans) and mold spores. It also breaks down the lipid envelope and proteins of viruses like influenza and coronaviruses.
● Sporicidal activity
Hydrogen peroxide also kills bacterial spores. Silver ions boost this effect by attacking spore-coat proteins, making the system effective against Bacillus and Clostridium spores.
● Effectiveness against biofilms
Silver-H₂O₂ penetrates dense biofilm layers on devices, pipes, and surfaces. Lab tests record sharp drops in biofilm mass and cell viability, while real-world trials report cleaner water lines, medical tools, and food-processing equipment.
Application Methods
Silver Hydrogen Peroxide disinfectants are manufactured with different concentrations and formulations. You must understand your cleaning needs and accordingly use application methods, such as:
- Surface-Level Spraying and Wiping: Dilute with water and use a spray bottle to apply on the cleaning surface.
- Fogging by Misting: A mist generator helps disperse fine droplets of disinfectant on areas out of your reach, such as narrow openings or ceilings.
- Aerial Disinfection (Cold Fogging/ULV): For aerial disinfection, use cold fogging and Ultra-low-volume (ULV) systems. It involves misting disinfectant particles and dispersing finer
- Water System Sanitization: Infuse Silver-H₂O₂ in your pipelines, tanks, and filters to kill biofilms and microbes.
- Material Compatibility: Check the recommended strength on metals, plastics, or fabrics to avoid corroding pipes, fading plastics, or weakening textiles.
Advantages Over Conventional Disinfectants
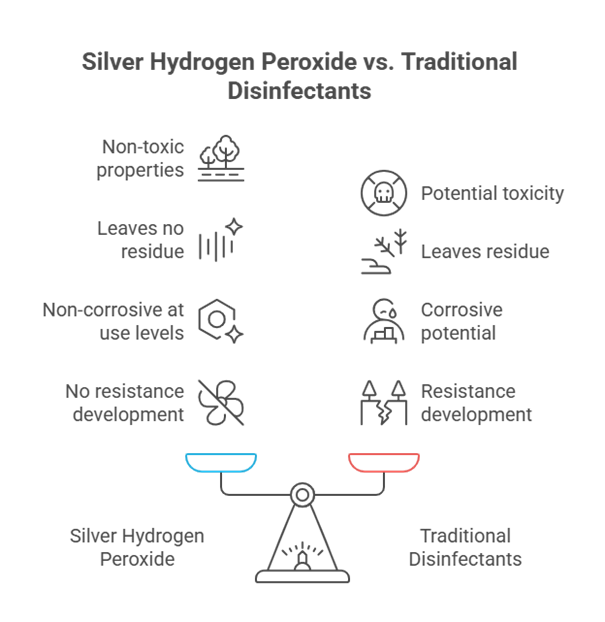
Fig. 1 Advantages of Silver Hydrogen Peroxide over traditional disinfectants
SHP-based disinfectants have various advantages over other chemical agents, like as chlorine and phenolics, including:
- Biodegradable and nontoxic: Silver-H₂O₂ is biodegradable as it produces no hazardous byproducts, residues, or chemical traces (except water and oxygen).
- Non-Corrosive at Use Levels: With lower (1.5-5%) concentrations of H2O2, this disinfectant is non-corrosive for metals and plastics.
- No Resistance Development: With oxidation and silver ions, it stays effective even after repeated usage, not letting the microbes adapt to it.
- Extended Action from Silver Ions: After peroxide evaporates, silver ions stay on the surface to eliminate existing microbes.
Safety and Handling Guidelines
As silver hydrogen peroxide is growing in popularity as a disinfectant across industries, you must adhere to these safe handling guidelines:
- Recommended Concentrations: For water systems, use 100-1,000 ppm H₂O₂ while cleaning and 25 ppm for maintenance. Use 1.5-5% of H₂O₂ with 15-50 ppm Ag⁺ for surface and aerial
- Personal Protective Equipment (PPE): You must use PPE and wear gloves, eye protection, and a respirator when handling the concentrate or fogging.
- First Aid and Storage Precautions: If exposed, rinse immediately and seek medical help if you ingest or inhale it. Store SHPs in sealed containers in a cool and dry spot away from sunlight and incompatible chemicals.
- Regulatory Compliance: You must follow the updated guidelines by the WHO and the US Environmental Protection Agency (EPA) for the safe use of silver-based disinfectants.
Use Cases and Case Studies
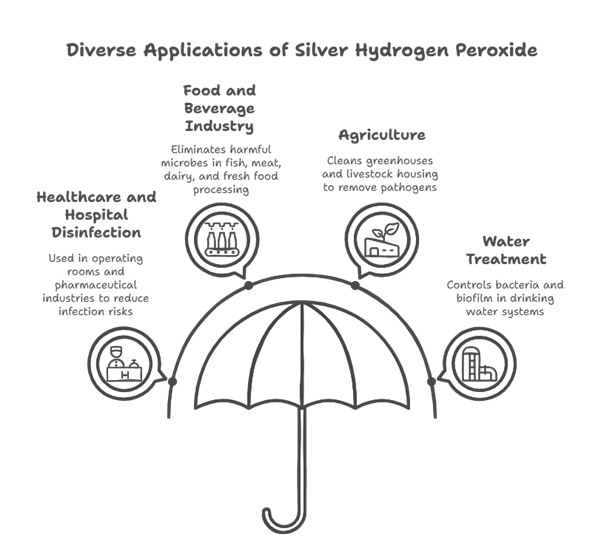
Given the broad-spectrum antimicrobial properties of silver hydrogen peroxide, it is widely used in industries like:
- Healthcare and Hospital Disinfection: It is colourless and odourless, and remains potent at various temperatures and pH levels. Therefore, effective for healthcare and pharmaceutical
- Food and Beverage Industry: It is useful in the fish, meat, dairy, and fresh food industry by eliminating harmful microbes like E. coli, Salmonella and Listeria.
- Agriculture (Greenhouse and Livestock Sanitation): Silver-H₂O₂ mist and fog in greenhouses and animal housing removes pathogens, cleans organic deposits and maintains clean water
- Water Treatment Applications: Treatment plants infuse SHPs in reservoirs and distribution lines to control bacteria and biofilm in drinking water systems.
Challenges and Considerations
Silver-H₂O₂ often costs more than chlorine or quats, which can limit its use in large operations. Other limitations that you must consider are:
- Shelf-Life and Degradation: The active ingredients of the SHPs may break down over time if the silver ions are in low concentrations.
- Light and Temperature Sensitivity: Heat and sun exposure also cause storage issues, making it harder to store for extended periods.
- Scale and Load Limits: For large-scale disinfection, industries require higher volumes, which can be challenging to store and maintain stable concentrations.
Future Trends and Research Directions
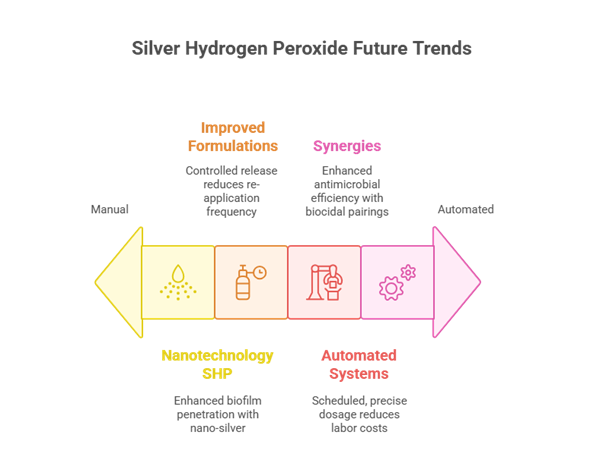
Silver hydrogen peroxide, which was discovered in 1818 and first extracted in 1894, has seen widespread usage since its inception. With growing technology, this is what the future of these SHPs looks like:
- Nanotechnology and SHP: SHPs can now be enhanced with nano silver ions and nanocarriers for better biofilm penetration.
- Improved Formulations with Slow-Release Properties: The improved SHP gels and coatings release active agents over days or weeks, reducing the need for multiple reapplications.
- Automated Disinfection Systems: SHP blends integrate with timed fogging and misting units to deliver exact doses on schedule, cutting labour costs by reducing the need for manual
- Synergies with Other Biocidal Technologies: Silver-H₂O₂ can now pair with UV irradiation (UV/H2O2), ozone, or biodegradable enzymes to improve its antimicrobial efficiency.
Silver Hydrogen Peroxide Disinfectant as an Eco-friendly Alternative
Silver hydrogen peroxide (SHP) is an effective, safe, and biodegradable disinfectant. By using silver’s catalytic capability and hydrogen peroxide’s oxidative nature, it eliminates all the superbugs, from bacteria to viruses. It is non-toxic, leaving behind only water and oxygen after application. The silver ions make the concentration stable, increasing its shelf life and lowering your labour and chemical costs. It is a multipurpose disinfecting solution with surface, water, and aerial usage. Finding its applications across industries, from healthcare to food processing, it is one of the most viable alternatives to other chemical agents.
References
- https://pubmed.ncbi.nlm.nih.gov/26154263/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8741583/
- https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen-Peroxide
- https://agriculture.vikaspedia.in/viewcontent/agriculture/agri-inputs/inorganic-inputs/crop- protection?lgn=en
- https://www.slideshare.net/slideshow/silver-hydrogen-peroxide/63976055
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7167925/
- https://medium.com/@SilverHydrogenPeroxide/silver-hydrogen-peroxide-disinfecting-the-ec o-friendly-way-9e176f1e9e97
- https://shelkaindustry.com/silver-hydrogen-peroxide/
- https://medicalresearchjournal.org/index.php/GJMR/article/view/947/3-Hydrogen-Peroxide- Silver_JATS_NLM_xml
- https://www.lenntech.com/processes/disinfection/chemical/disinfectants-hydrogen-peroxid htm
- https://www.quora.com/Why-isn-t-hydrogen-peroxide-commonly-used-as-a-disinfectant-for- the-home-Not-exaggeration-it-is-literally-the-only-thing-that-is-non-toxic-to-humans-and-ani mals-turns-to-water-after-exposed-to-the-air-for-10-minutes
- https://www.comfortservicesgroup.co.uk/silver-peroxide-water-treatment/
- https://www.researchgate.net/publication/273686211_Antibacterial_effects_of_Hydrogen_ peroxide_and_silver_composition_on_selected_pathogenic_enterobacteria
- https://www.cdc.gov/infection-control/media/pdfs/guideline-disinfection-h.pdf#page=46.39
- https://www.researchgate.net/publication/274826831_Efficiency_evaluation_of_the_disinfe ctant_based_on_silver_and_hydrogen_peroxide