Related Technical Articles
From PPD to PTD: The Evolution of Safe Hair Dye Chemistry
Hair dyeing is among the most common cosmetic practices worldwide- a process deeply rooted in both fashion and science. Hair dyeing involves a chemical reaction that alters the internal structure of a hair strand. In permanent dyes, small precursors enter the cortex, oxidise in situ, and couple to form large coloured molecules that remain locked within the fibre. The chemistry behind permanent hair dye involves controlled oxidation reactions that create long-lasting colors inside the hair shaft.
Recent data highlight how widespread this practice has become. About 40% of Indian adults now colour their hair, whereas in Europe and the United States, around 33% of women and over 10% of men do the same. Such widespread use makes understanding dye chemistry & its toxicological safety crucial for both formulators and consumers.
One of the earliest and most effective precursors, p-phenylenediamine (PPD), produces deep, stable shades but is also a potent contact allergen. It’s safer analogue, ptoluenediamine sulfate (PTD), offers similar colour performance with reduced sensitisation risk.
This article explores the science behind this transition- from the molecular reactivity of PPD to the safer, next generation chemistry of PTD highlighting how small structural modifications can transform product safety without compromising color performance
Background: The Role of Oxidative Dyes in Hair Colouring
Permanent hair dyes rely on oxidative chemistry. Oxidative dyes are the key components of permanent hair colouring. They offer unmatched durability and colour range. Let us discuss their chemical foundations, mechanisms, and the role of dye precursors required in creating long-lasting, customisable results:
Types of Hair Dyes and Site of Action
Hair dyes are generally classified by mechanism and durability: direct or temporary dyes (8– 12 washings), semi-permanent (∼24 washings), and oxidative or permanent dyes (until hair grows out).
- Direct dyes deposit pre-formed pigment molecules onto the surface or shallow layers of the cuticle. They require no chemical activation and wash out within a few shampoos.
- Semi-permanent dyes penetrate partially; lasts about 20-24 washes.
- Oxidative dyes, by contrast, are colourless precursors that diffuse into the hair cortex, which is the dense inner layer of the hair shaft. Once reach inside, they undergo an oxidative coupling reaction to form large coloured molecules too large to leach out.
Chemical Mechanism of Oxidative Dyeing
The oxidative dyeing process occurs in two steps: oxidation of a primary intermediate, followed by coupling with a colour modifier (coupler). This reaction is usually carried out in the presence of hydrogen peroxide (H₂O₂) and an alkaline agent like ammonia (NH₃) or monoethanolamine (C₂H₇NO).
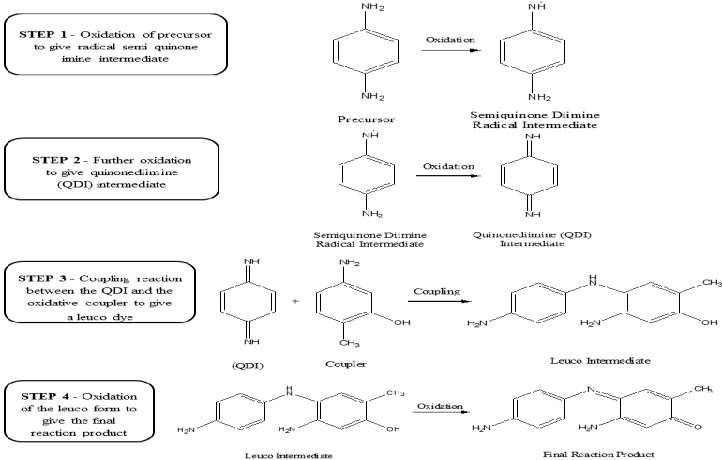
Figure 1. Stepwise oxidation and coupling mechanism of p-phenylenediamine (PPD), Adapted from Yildirim et al., 2022-
Oxidation of the primary intermediate
The primary intermediate, usually an aromatic diamine, undergoes oxidation by H2O2 in an alkaline medium to yield a reactive quinonediimine intermediate, which acts as an electrophile.
-
Coupling Reaction
This intermediate reacts with a coupler (such as resorcinol or m-aminophenol) to form a large, conjugated dye molecule. These polymeric chromophores are hydrophobic and become trapped within the keratin matrix. These characteristics prevent them from escaping the cortex.
This chemistry yields a durable, irreversible colour change that endures multiple wash cycles and environmental exposures.
-
Oxidation of the primary intermediate
Role of Intermediates and Couplers
Oxidative dyes are designed as two-component system:Component Function Examples Primary intermediates Undergo oxidation to form
reactive intermediates• p-Phenylenediamine (PPD)
• Toluene-2,5-diamine (PTD)
• ME-PPD (2-methoxymethylPPD)Couplers Defines final hue and tone • Resorcinol → brown/red
• m-Aminophenol → violet/red
• 1-Naphthol → golden/yellowPerformance Advantages of Oxidative Dyes
Oxidative dye systems remain the benchmark for permanent hair colouring, primarily because their chemistry delivers depth, precision, and endurance unmatched by other dye classes. Their structural integration explains their exceptional stability and versatility. The key advantages of using these dyes are as depicted in Figure 2:
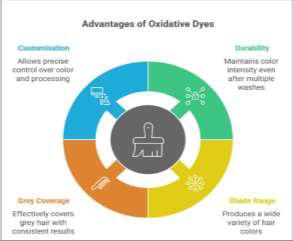
Figure 2. Advantages of Oxidative DyesDurability
Once the dye molecules form inside the cortex, they polymerise into large, hydrophobic chromophores that cannot diffuse out easily. The colour fades mainly because of external abrasion or UV-induced cuticle wear, not from washing out. Studies have shown that oxidative colours can maintain over 85% intensity even after 24 wash cycles when properly formulated.
Shade Range
Through variations in primary intermediates, such as PPD and PTD, and couplers like resorcinol (C₆H₄(OH)₂) or m-aminophenol (C₆H₇NO), manufacturers can produce virtually any tone. For example, deep black, rich brown, warm auburn, or cool ash. Subtle adjustments in coupler ratios fine-tune hue and reflectivity.
Grey Coverage
The small molecular size of oxidative precursors allows them to penetrate the compact, resistant cuticle of grey hair effectively. Inside, their rapid oxidation produces full chromatic saturation. Therefore, PPD- and PTD-based formulations are particularly valued in professional salons for their consistent grey coverage and minimal patchiness.
Customisation
Oxidative systems offer unparalleled control. Formulators can modify H₂O₂ concentration, alkalinity (via NH₃ or MEA), and dye ratios to influence lift, tone, and processing time. This adaptability enables both high-lift blonding and subtle tone correction within the same chemical framework.
The Chemistry of PPD (p-Phenylenediamine)
PPD (C₆H₈N₂) is an aromatic diamine containing two amino groups at parapositions on a benzene ring (Fig.3).
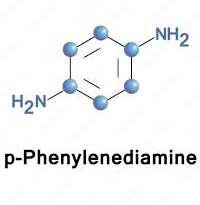
Figure 3. Chemical structure of (p-Phenylenediamine)This simple structure gives PPD its distinct reactivity. The para position of the amino groups allows symmetrical oxidation, which makes it an efficient base for oxidative dyes. In its pure form, PPD is a white crystalline solid. When exposed to air, it darkens due to surface oxidation, which is a sign of its chemical activity. This symmetrical structure gives PPD its strong reactivity & excellent color depth.
Structure and Reactivity
PPD’s reactivity lies in its amino groups. They act as nucleophiles and readily engage in oxidation and coupling reactions. The compound’s low molecular weight (108 g/mol) allows deep penetration into the hair color. Upon oxidation with H2O2, it forms a quinonediimine that couples with phenolic agents like resorcinol to yield deep brown or black pigment. Its para orientation also stabilises the reactive intermediates that form during oxidation. This stability helps maintain consistent colour results across different formulations.
Oxidation Mechanism
The dyeing process begins when PPD is mixed with H₂O₂ under alkaline conditions (pH 9 - 10), usually created by NH₃ or monoethanolamine. PPD undergoes oxidation to form a quinonediimine intermediate.
- The intermediate is unstable and reacts almost immediately with couplers to produce complex, conjugated dye molecules.
- These molecules polymerise inside the hair shaft and become trapped within the keratin structure.
Colour Characteristics and Performance
Depending on coupler selection, PPD can produce:
- With resorcinol → warm brown or red-brown tones
- With m-aminophenol → cooler brown to blue-black tones
These polymeric pigments absorb light primarily between 470-550nm, giving the natural, long-lasting hues desired in permanent hair color.
Limitations and Health Concerns
PPD’s strong reactivity also underlines its allergenic nature. During oxidation, it forms quinonediimine intermediates that can react with skin proteins. These intermediates, and their byproducts such as Bandrowski’s base (C₁₈H₂₁N₆), are known to trigger allergic contact dermatitis (ACD) in sensitive individuals. Symptoms range from scalp erythema and itching to facial oedema and vesiculation. Patchtest studies report sensitisation in approximately 1.5% of the general population and up to 20% of hairdressers exposed occupationally. To mitigate risk, regulatory agencies have imposed concentration limits and mandatory labelling:
- European Union: ≤ 6% in oxidative dye mixtures (with couplers)
- India (BIS): ≤ 4% after dilution for consumer use
- United States (FDA): Permitted for scalp application with patch-test warnings
The Shift Toward p-Toluenediamine Sulfate (PTD)
The single methyl group at the ortho-position changes the molecules reactivity, making it less aggressive towards biological tissues while maintaining effective oxidation behaviour.
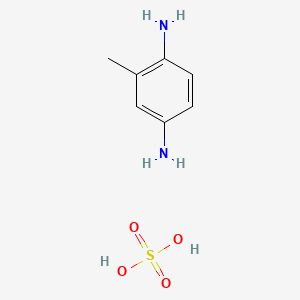
Figure 4. Chemical Structure of p-Toluenediamine Sulfate (Adapted from PubChem)This seemingly minor substitution has profound implications for both dye chemistry and toxicology. It makes PTD one of the most widely adopted “low-sensitisation” alternatives in modern permanent hair colour formulations.
Structural and Chemical Differences
The chemical distinction between p-phenylenediamine (PPD) and p-toluenediamine (PTD) lies in a single methyl substitution that alters the molecule’s reactivity, stability, and biological profile. The following Table 1 summarises the key structural and chemical differences that define this shift and explain PTD’s lower allergenic potential.
Table 1. Structural and Chemical Comparison of PPD and PTD
Property p-Phenylenediamine (PPD) p-Toluenediamine (PTD) Remarks / Implications Chemical name 1,4-Diaminobenzene 2-Methyl-1,4-diaminobenzene PTD is a methylated analogue of PPD Molecular formula C₆H₄(NH₂)₂ C₇H₁₀N₂ PTD contains one additional carbon and two hydrogens due to the –CH₃ group Molecular weight 108.14 g/mol 122.17 g/mol Slightly heavier; affects solubility and diffusion rate Functional groups Two amine groups (–NH₂) Two amine groups (–NH₂) and one methyl group (–CH₃) The methyl group modifies electron density and reactivity Position of substituents Amines at para (1,4) positions Amines at 1,4 with a methyl at the 2-position Ortho-methyl introduces mild steric hindrance Electron density Uniform across the aromatic ring Slightly increased near ortho position; lower availability for oxidation The methyl group reduces overall electrophilic activity Oxidation behavior Rapid oxidation to quinonediimine intermediates Slower oxidation; produces larger, less reactive intermediates Leads to fewer protein-reactive byproducts Typical oxidation product Quinonediimine → Bandrowski’s base (C₁₈H₂₁N₆) Toluenediamine → stable indoaniline dye PTD forms fewer allergenic side products Water solubility Moderate (~37 g/L) Higher as sulfate salt (~72 g/L) PTD sulfate is easier to formulate in aqueous bases Color outcome Intense dark brown to black Similar depth, slightly warmer or softer tone PTD yields a more natural appearance Relative sensitisation risk High Lower Methyl group limits protein binding; reduced allergenic potential Characteristics compared from PubChem
Oxidation and Coupling Mechanism
PTD follows the same fundamental oxidative pathway as PPD: the amine groups are oxidised by H₂O₂ in an alkaline medium to form quinonediimine intermediates. However, the methyl substituent at the ortho position introduces mild steric hindrance, slowing the rate of oxidation and coupling.
The reaction goes like this:
- C₇H₁₀N₂ + H₂O₂ → C₇H₈(NH)(NH)=NH₂ (Toluenediimine intermediate)
- Toluenediimine + Coupler → Diphenylamine or Indoaniline dye + H₂O
The intermediates formed are slightly larger and less reactive toward skin proteins, yet still reactive enough to combine with couplers like resorcinol or m-aminophenol to produce deep brown to black pigments. As the methyl group’s electron-donating effect, PTD oxidation generates pigments with marginally warmer undertones, often perceived as softer or more natural compared to the denser tone of PPD-based dyes.
Colour Outcomes and Aesthetic Properties
PTD undergoes the same general oxidation and coupling mechanism as PPD but at a slower rate due to steric hinderance from the methyl group.
C7H10N2 + H2O2 Toluenediimine Coupler reaction Indoaniline dye
The resulting pigments exhibit slightly warmer undertones perceived as more natural and less intense than PPD based shades.
Toxicological and Allergenic Advantages
The main reason PTD replaced PPD in many formulations is its lower sensitisation risk. A single methyl group alters electron distribution and slightly reduces oxidation speed. This change limits the formation of reactive intermediates that bind to skin proteins and trigger allergies.
Clinical evidence shows clear improvements:
- Recent U.S. surveillance (2019–2022) showed PTD positivity of 1.6–1.7%, which is significantly lower than historical rates for PPD.
- A retrospective study of 8,036 patch-tested patients found 231 sensitised to PPD and 109 to PTD, confirming PTD’s lower allergenic rate.
- Toxicological models calculate a Margin of Safety (MOS) of 5.9 for PTD versus 2.7 for PPD, confirming a safer exposure margin under normal use.
These results make PTD a lower-risk alternative, though not risk-free. Dermatology experts and the SCCS agree: anyone sensitised to aromatic diamines should still avoid PTDcontaining products, as cross-sensitisation remains possible.
Conclusion
The transition from p-phenylenediamine (PPD) to p-toluenediamine sulfate (PTD) represents a defining step in the evolution of modern hair dye chemistry. It reflects a deliberate shift from pure performance toward a more balanced approach with colour longevity, user safety, and toxicological responsibility in equal measure. PTD’s molecular design preserves the deep, stable tones consumers expect while substantially reducing sensitisation risks. This advancement shows how small structural changes can meaningfully improve human compatibility without compromising aesthetic results. However, the work is far from complete. Continuous innovation in dye intermediates, green formulation chemistry, and regulatory science will remain essential to achieve safer and more sustainable cosmetic colourants!
About NICHEM
Long-standing Specialty Chemicals player with ISO 9001:2015 certification and a history of providing specialty solutions for over 25 years. The company is headed by senior chemical industry specialists with the combined expertise of more than 100 years. With an emphasis on eco-friendly, non-toxic products, the company’s primary strength is research, development, and customization. More information on NICHEM can be found at https://nichem.solutions.
References:
Mintel. (2025, May 8). India Hair Colourants Market Report 2024 | Mintel Store. Mintel Store.
https://store.mintel.com/report/india-hair-colourants-market-report
PubChem. (n.d.). 2,5-Diaminotoluene sulfate. PubChem.
https://pubchem.ncbi.nlm.nih.gov/compound/22856#section=2D-Structure
News-Medical. (2025, August 6). Health Implications of p-Phenylenediamine: The Aromatic Amine in Permanent Hair Dye.
https://www.news-medical.net/health/Health-Implications-ofpe28091Phenylenediamine-The-Aromatic-Amine-in-Permanent-Hair-Dye.aspx
Hair Dye Alternative Named Allergen of the Year. (n.d.).
https://www.medscape.com/viewarticle/hair-dye-alternative-named-allergen-year2025a10005p1
Vogel, T. A., Heijnen, R. W., Coenraads, P., & Schuttelaar, M. L. (2016). Two decades of p‐phenylenediamine and toluene‐2,5‐diamine patch testing – focus on co-sensitisations in the European baseline series and cross‐reactions with chemically related substances. Contact Dermatitis, 76(2), 81–88.
https://doi.org/10.1111/cod.12619
Gargano, E. M., Blömeke, B., Gaspari, A. A., & Goebel, C. (2022). The 2-Methoxymethyl modification of P-Phenylenediamine reduces the sensitisation risk for hairdressers to hair Dyes—An Occupational Hand Exposure–Based Risk Assessment. Dermatitis, 33(4), 293–301.
https://doi.org/10.1097/der.0000000000000915