Related Blogs
Iodine: An Antimicrobial Element
Iodine is one of the most essential micronutrients for the human body. It is a naturally occurring halogen that is often used in industrial sectors to disinfect contaminated surfaces and water treatment. Today, the challenges of antimicrobial resistance developed by pathogens have made many traditional disinfectants ineffective. To overcome these challenges, iodine’s oxidative mechanism makes it a broad-spectrum agent that kills bacteria and viruses without letting them develop resistance.
To make it more effective, it is used with additives and delivered through controlled systems. This guide will explore why iodine is a good alternative to chlorine and silver as a sustainable solution across sectors like food, water, and healthcare.
Chemical Nature of Iodine
Iodine (I) is a member of the halogen family (Group 17) that occurs naturally in seawater. It remains solid at room temperature and pressure. Iodine is present as violet-black crystals that evaporate into a violet-coloured vapour. This chemical reactivity in its oxidative state makes it a mild oxidising agent compared to traditional disinfectants like chlorine.
In antimicrobial technology, Iodine is not used in raw form due to its volatile nature and solubility challenges. Hence, it is used through different chemical forms such as:
- Elemental Iodine (I2): An active biocidal species, though highly volatile in its pure state.
- Iodophores: Povidone-Iodine (PVP-I) is a complex where iodine is bound to a polymer carrier. It acts as a reservoir that releases “free” active iodine for better stability.
- Iodinated Resins: These solid-phase delivery mediums bind iodine to anion-exchange resins. In water treatment systems, this system releases iodine only when a microorganism comes in contact.
- Iodine-Releasing Complexes: These formulations use amino acids or surfactants (example: charge-transfer complexes with polysaccharides or cyclodextrins).
The Harmful Side – When Microbes Turn Dangerous:
Not all microbes are good for you. Some can make you sick. These harmful microbes are called pathogens.
- Bacteria: Some types cause diseases like tuberculosis, pneumonia, and food
- Viruses: These can lead to illnesses such as influenza, HIV/AIDS, COVID-19, and the common
- Fungi: They can cause conditions like athlete’s foot, ringworm, and serious infections in people with weak immune systems.
- Protozoa: These include parasites that cause malaria and other tropical
Microbes affect both industries and public health. Some can spoil food, contaminate water, and spread in healthcare facilities. You need to manage them carefully because they can cause problems on a global scale.
Mechanism of Antimicrobial Action
Iodine has the ability to work as a protoplasmic poison against microbes by penetrating their cell walls and membranes. Inside the cell, it acts as an oxidising agent, such as the sulfhydryl (-SH) groups in cysteine and methionine (essential amino acids) and phenolic groups. This disrupts the electron transport chain used by cells to produce energy and their metabolic system.
Iodine also affects nucleic acids (DNA and RNA) and fatty acids within the cellular structure, leading to membrane destabilisation. This provides clinical and industrial advantages such as:
- Broad-Spectrum Efficacy: It is effective against Gram-positive and Gram-negative bacteria, viruses, fungi, protozoa, and even resilient bacterial spores.
- Low Resistance Development: iodine can attack multiple cellular targets (cell membrane, cytoplasm, and nuclear material) simultaneously, rather than a single metabolic pathway. Hence, microbes cannot easily develop genetic resistance.
- Compatibility: Iodine is compatible with most of the additives to create formulations with surfactants and stabilisers. These enhance its ability to penetrate biofilms, ensuring that the active agent reaches various microbes.
Role of Speciality Additives in Iodine Optimisation
To stabilise iodine and enhance its performance, additives are used to minimise its volatile nature. These include polymers, surfactants, or carriers such as polyvinylpyrrolidone. The additives create weak bonds with hydrogen to hold the iodine molecule, making it stable and increasing its shelf-life.
These additives optimise the delivery of iodine through:
- Controlled and Sustained Release: By controlling the free iodine within a carrier, additives gradually release iodine, rather than releasing it too fast.
- Improved Solubility and Dispersion: Naturally occurring iodine has poor water solubility (0.03 g/100 mL at 20°C). To make it more soluble, additives help it disperse uniformly in the aqueous solution.
- Reduced Sensory Impact: Additives reduce the strong odour and the brown stains of iodine. This makes it suitable for consumer, healthcare, and food-related uses.
Challenges with Conventional Use
Even though iodine is an efficient disinfectant, using it in its raw form poses a few physical and chemical limitations. The free iodine has a high vapour pressure, increasing its volatility, which leads to fast depletion. This means its active concentration vanishes during storage or from a surface. These factors reduce its overall ability to protect against pathogens and increase the risk of inhalation in dynamic usage.
The other operational and safety limitations are:
● Staining and Odour
Traditional iodine solutions may leave a brownish stain on fabrics, plastics, and other porous surfaces. They can also corrode metals in acidic environments. It also gives off a medicine-like odour, making it inappropriate for use in textile or equipment-heavy sectors.
● Dose Control
Over-iodination can result in chemical residues that may be dangerous to health. At the same time, underdosing may not be potent enough to kill microbes. It is difficult to maintain an optimum window in real-time applications without timed delivery systems.
● Limited Persistence
In dynamic systems like water lines, iodine gets washed away easily. Moreover, on surfaces that often come in contact with food, it may get neutralised by organic matter.
Advanced Iodine Additive Technologies
Today, modern additives are used to manage the volatile nature and handling challenges of iodine. These make it stable and release it through systems like iodophores, polymers, and encapsulated platforms, as discussed below:
Iodophores and Complexed Iodine Systems
The development of iodophores enhances the iodine chemistry through:
- Controlled Iodine Availability: It stores the bulk of iodine and releases free molecular iodine as required for microbial activity. This keeps it from depleting fast during application.
- Improved Stability: They work with carriers like polyvinylpyrrolidone (PVP) to reduce the vapour pressure of iodine. This reduces loss due to the sublimation activity of iodine and makes it more stable.
- Performance Tuning: These systems use surfactants to release the measured ratios of iodine per application.
Polymer- and Resin-based Iodine Additives
Iodine is used with polymer or ion-exchange resins for applications that need solid-phase antimicrobials. These help with:
- Immobilised Iodine: The resin holds iodine in place by keeping it stuck with charged sites called quaternary ammonium. Hence, it releases iodine only when any pathogen comes in contact with the medium, killing it immediately.
- Predictable Release Kinetics: modern additives like polyurethane and chitosan-based systems help with the predictable release of iodine in the treatment plants. This creates a consistent barrier, where free iodine is released only when its amount in the present solution has reduced.
- Low Leaching: By creating a coating of polymer or resin, these can be effectively used to filter water and reduce the leaching of chemicals in the ecosystem.
Encapsulation and Sustained-release Platforms
Iodine may degrade due to UV light exposure, pH fluctuations, and volatilisation. Encapsulation technology prevents this through:
- Microencapsulation: They cover iodine with semi-permeable shells (cyclodextrins or specialised polymers), creating a barrier from moisture or pH changes.
- Protection: These protect iodine from light and heat exposure so that it can be used in applications that require thermal processing or long shelf life.
- Targeted Delivery: They help deliver iodine into the food industry or wastewater treatment, where free iodine may be neutralised. One can also use it in the health sector to disinfect wounds or contaminated sites.
Spectrum of Applications
The stabilised iodine is versatile for a wide range of antimicrobial applications across industries. These include:
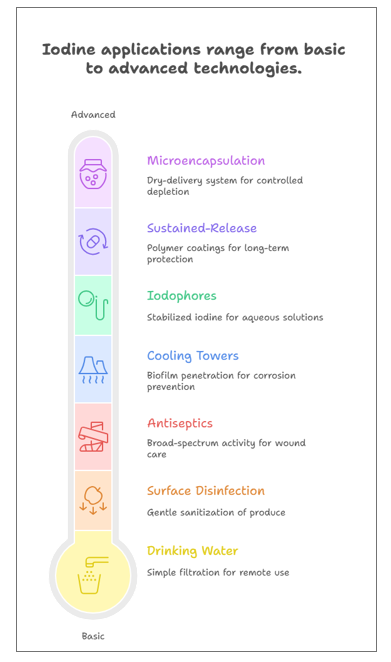
Figure 1. Applications of Iodine
Water Treatment and Disinfection
Iodine is used in different delivery systems to disinfect the drinking water and kill sessile bacteria:
- Drinking Water: Many remote communities and space missions by NASA use resin-based iodine filters for their point-of-use water systems. They do not require additional equipment or electricity to set up.
- Biofilm Control: The natural charge of iodine helps it penetrate the layers of biofilm in pipelines and storage tanks.
Food and Beverage Safety
To sanitise products in the food industry, iodine works as a gentle disinfectant with:
- Surface Disinfection: It helps sanitise the surfaces of fruits, vegetables, and seafood. Unlike chlorine, iodine works efficiently in the presence of organic matter (dirt/soil).
- Equipment Hygiene: Iodine disinfectants do not corrode stainless steel or show the buildup of mineral scales. That is why these work best for dairy and beverage processing plants.
Healthcare and Pharmaceutical Uses
Iodine displays a broad-spectrum activity, making it ideal for use in antiseptics and other medical uses:
- Antiseptics and Wound Care: Povidone-Iodine (PVP-I) is used for disinfecting surgical scrubs and the pre-operative skin. The new technology of cadexomer iodine dressings slowly releases iodine on wounds to heal them without harming the underlying tissues.
- Implant Coatings: These release iodine on titanium implants to keep any infection from spreading after a surgery.
Industrial and Environmental Applications
Various industries use iodine to protect the machinery while being environment-friendly disinfectant:
- Cooling Towers: Iodine can easily penetrate the biofilm and algae with automated dosing delivery. It works effectively in alkaline water, preventing microbial-induced corrosion (MIC), unlike chlorine.
- Antimicrobial Coatings: For Heating, Ventilation, and Air Conditioning (HVAC) systems and public transport, these coatings help provide surface disinfection.
Iodine Delivery Technologies
The advanced stabilisers and delivery systems help overcome the challenges of free iodine, giving the following benefits:
- Overcoming Limitations: Iodophores (PVP-I) stabilise the elemental iodine for application in aqueous solutions in the health and food industries.
- Sustained-Release: Polymer and resin-based coatings help protect filters and implants for long-term use by releasing iodine consistently and gradually.
- Future Tech: Microencapsulation is gaining popularity with its dry-delivery system and packaging that keeps iodine from depleting too fast.
Safety, Toxicology, and Regulatory Considerations
Iodine is one of the most important micronutrients for human health. Overdosing or underdosing needs to be regulated by defining the correct concentration for various industries. This helps prevent toxicity and associated health issues like thyroid dysfunction.
The National Research Council established a Recommended Dietary Allowance (RDA) of 150 µg per day for most adults. However, the World Health Organisation (WHO) has set a maximum daily intake of 1 mg from all sources.
Regulatory agencies like the EPA and FDA have these guidelines for iodine concentrations in the environment and industries:
- Drinking Water: 15 µg/L for long-term consumption.
- Food Contact: For salt iodisation, its use must be approximately 01% in table salt.
- Industrial Systems: Higher concentrations are allowed in cooling towers and non-potable water systems (5-10 ppm) to ensure biofilm penetration.
Performance Advantages Over Conventional Biocides
If compared with chlorine, silver, and quaternary ammonium compounds (QACs), iodine is a better alternative with better protection against a broader spectrum of pathogens. The table below summarises the differences between each of these disinfectants:
Biocide | Primary Mechanism | Resistance Risk | Limitations |
Iodine | Multi-target oxidation | Very Low | Volatility and staining |
Chlorine | Membrane/Protein damage | Moderate | High reactivity; pH sensitive |
Silver | Thiol-group binding | Low-Moderate | Slow kill rate; high cost |
QACs | Membrane disruption | High | Environmental persistence |
Peroxides | Free radical oxidation | Low | Highly unstable; corrosive |
The advantages of using iodine are:
- Broad Spectrum and Resistance: QACs or silver can induce resistance (e.g., efflux pumps). However, iodine’s mechanism of protein oxidation makes it immune to the development of microbial resistance.
- Balanced Efficiency: It has a better kill rate than silver and is more stable than chlorine.
Despite these advantages, the volatility, strong odour, and staining of iodine limit its usage. These can be mitigated by creating an iodine complex with surfactants (PVP or non-ionics). The concentration of free iodine is kept low enough to kill pathogens without staining the surface.
Sustainability
The use of iodine-based disinfectants has become popular with the advancement in green chemistry. They utilise a natural biogeochemical cycle with modern delivery mechanisms that are environment-friendly.
Most of the synthetic biocides accumulate in the ecosystems. However, iodine occurs naturally and is biodegradable by microbial processes. This happens through the cycle of oxidation and reduction, converting it into iodide and reducing its overall carbon footprint.
Today, its use has become even more sustainable with the help of smart systems:
- The additives used in iodine ensure its sustained and uniform release, keeping it from accumulating in the environment.
- The polymer-based microencapsulation and resin filters are reusable materials, creating less waste.
- By using stabilised formulations, the release of volatile iodine gas can be reduced into the troposphere.
Conclusion
Iodine, as an antimicrobial agent, works efficiently without causing any operational or environmental risks. Through its oxidative process, it showcases the property of broad-spectrum disinfection without letting pathogens develop any resistance. The modern delivery systems, such as iodophores or polymer-based coatings, address the challenges of volatility, stability, and staining. This makes it one of the most potent disinfectants for industrial sectors, healthcare usage, and water and food treatment. Today, it has become a versatile, sustainable, and future-proof antimicrobial agent that causes minimal harm to human health and the environment.
About NICHEM
Long-standing Specialty Chemicals player with ISO 9001:2015 certification and a history of providing specialty solutions for over 25 years. The company is headed by senior chemical industry specialists with the combined expertise of more than 100 years. With an emphasis on eco-friendly, non-toxic products, the company’s primary strength is research, development, and customization. More information on NICHEM can be found at https://nichem.solutions.